QM/MM studies reveal pathways leading to the quenching of the formation of thymine dimer photoproduct by flanking bases†
Abstract
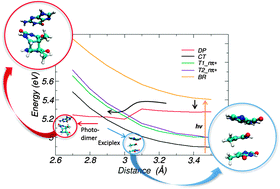
It is known that the formation of the photochemical product of thymine–thymine cyclobutane pyrimidine dimer (TT-CPD) formed upon UV excitation in DNA is significantly affected by the nature of the flanking bases, and that the oxidation potential of the flanking base correlates with the quenching of TT-CPD formation. However, the electronic details of this correlation have remained controversial. The quenching of thymine dimer formation exerted by flanking bases was suggested to be driven by both conformational and electronic effects. In the present study, we examine both of these effects using umbrella sampling and a quantum mechanical/molecular mechanical (QM/MM) approach for selected model systems. Our results demonstrate that a charge transfer (CT) state between the flanking base and the adjacent thymine base can provide a decay pathway for the population to escape from dimer formation, which eventually leads to the formation of an exciplex. The QM/MM vertical excitation energies also reveal that the oxidation potential of flanking bases correlates with the energy level of the CT state, thereby determining whether the CT state intersects with the state that can lead to dimer formation. The consistency between these results and experimentally obtained dimer formation rates implies that the quenching of dimer formation is mainly attributed to the decay pathway via the CT state. The present results further underline the importance of the electronic effects in quenching.

 Please wait while we load your content...
Please wait while we load your content...