LEIS and XPS investigation into the growth of cerium and cerium dioxide on Cu(111)
Abstract
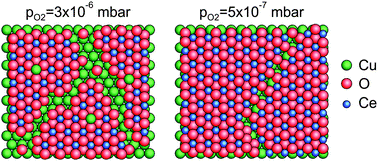
The controlled growth of Ce and CeO2 on Cu(111) was investigated applying low energy ion scattering spectroscopy (LEIS) and X-ray photoelectron spectroscopy (XPS). Previous LEIS studies on metallic and oxidised cerium deposits using other metallic substrates reported serious difficulties related to the neutralization of noble gas ions. For this reason, special attention was paid here to reveal possible matrix effects for the neutralization (“neutralization effects”), which would severely hinder quantitative evaluation of the LEIS data. The adsorption of O2 on Cu(111) induced no neutralization effects either with He+ or Ne+. Similarly, no neutralization effects were identified using He+ upon the deposition of metallic Ce on Cu(111), but it arises for the Ce peak monitored with Ne+. The initial growth of Ce is two dimensional up to ΘCe ∼ 0.5 ML, while almost complete coverage of Cu(111) is achieved at ΘCe = 2 ML. CeO2(111) was deposited evaporating Ce in a background of O2 at a sample temperature of 523 K. No neutralization effects were observed either with He+ or Ne+. In harmony with literature data, the growth mode is three dimensional. Here it was demonstrated that the continuity of the film, which could be efficiently checked by LEIS, is influenced by the applied oxygen pressure in the range of 5 × 10−7–3 × 10−6 mbar. At pO2 = 3 × 10−6 mbar the film was not completely closed even at relatively large coverages (16 ML), and a significant part of copper atoms were oxidized to Cu1+. Deposition of CeO2 at pO2 = 5 × 10−7 mbar was characterized by a nearly perfect wetting, with metallic copper atoms at the interface, and with a slightly more reduced ceria layer.

 Please wait while we load your content...
Please wait while we load your content...