A theoretical study on photophysical properties of triphenylamine-cored molecules with naphthalimide arms and different π-conjugated bridges as organic solar cell materials†
Abstract
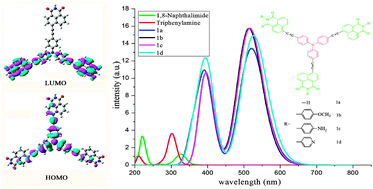
A series of D–π–A star-shaped molecules with triphenylamine (TPA) as a core, 1,8-naphthalimide (NI) derivatives as end groups, and different π-bridges have been designed to explore their optical, electronic, and charge transport properties as organic solar cell (OSC) materials. The calculation results showed that the star-shaped molecules can lower the material band gap and extend the absorption spectrum towards longer wavelengths. The designed molecules own the longest wavelength of absorption spectra, oscillator strength, and absorption region values. Our results suggest that the designed molecules are expected to be promising candidates for OSC materials. Additionally, the molecules with ethyne, thiophene, benzo[c][1,2,5]thiadiazole (BTA), and 2,3-dihydrothieno[3,4-b][1,4]dioxine (DTD) as π-bridges and 4-pyridne, 4-aniline, and H in NI fragments have better hole- and electron transporting balance and can act as nice ambipolar materials. The values of hole mobility of molecules with ethyne as a π-bridge and NI as an end group for Pna21 and P21/c are 5.30 × 10−3 and 1.27 × 10−2 cm2 V−1 s−1, respectively. On the basis of the investigated results, we suggest that molecules under investigation are suitable donors of [6,6]-phenyl-C61-butyric acid methyl ester (PCBM) and its derivatives are acceptors of solar cells.

 Please wait while we load your content...
Please wait while we load your content...