Simulating growth morphology of urea crystals from vapour and aqueous solution
Abstract
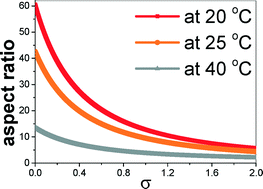
The molecular-scale understanding of the mechanism through which solvents interact with crystal surfaces during crystal growth processes is still a formidable challenge. Understanding this mechanism quantitatively is of paramount interest to the chemical and pharmaceutical industries, for controlling the evolution of growth morphologies. Here, we present a crystal growth model to elucidate the effect of internal and external growth parameters, solute, solvents, and their corresponding concentrations, to predict the growth morphology of crystalline urea from vapour and aqueous solution. The approach is based on details of the thermodynamic and kinetic aspects of adsorption of solute and solvent molecules at crystal faces; thus, relating these to crystallographic information, different solid–liquid interfacial energetics and external growth parameters affecting the rate of growth can be taken into account in a natural way. The binding energy of the solute molecules on the crystal surfaces is described by the molecular attachment energies which, in turn, depend on the molecular orientation and surface relaxation of the habit faces. A periodic first-principles method has been employed for accurate determination of solid–solid and solid–liquid energetics, which have been further utilized to calculate the solvent-induced step energy and local dissolution enthalpy of solute molecules at various faces of the urea crystal. Our results show that the step energy decreases with increasing supersaturation and temperature. On the other hand, an increase in supersaturation increases the effective concentration and local dissolution enthalpy of the urea molecule in aqueous solution. The rates of growth of different faces of the urea crystal, as a function of the driving force at various temperatures, have been determined within the framework of the discussed model, and the growth morphologies have been obtained. Our results show the appearance of block and needle-like habits of the urea crystals from vapour and from aqueous solution, respectively, as functions of supersaturation and temperature, in excellent agreement with corresponding experimental observations.


 Please wait while we load your content...
Please wait while we load your content...