Effects of hydroxy substituents on Cu(ii) coordination polymers based on 5-hydroxyisophthalate derivatives and 1,4-bis(2-methylimidazol-1-yl)benzene†
Abstract
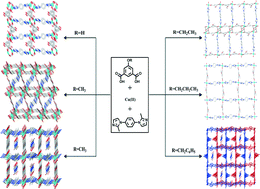
In order to systematically investigate the effects of 5-positioned substituents in isophthalates on the structures and properties of coordination polymers, six Cu(II) coordination polymers, [Cu2(bmib)(HO–ip)2]n (1), [Cu4(bmib)(OH)2(MeO–ip)3]n (2), [Cu(bmib)(MeO–ip)]n·(DMF)2n·(H2O)2n (3), [Cu(bmib)0.5(H2O)(EtO–ip)]n·(DMF)0.5n (4), [Cu(bmib)(PrO–ip)]n·(H2O)2.5n (5) and [Cu(bmib)(BnO–ip)]n·(DMF)n (6) have been synthesized by hydrothermal reaction of 5-hydroxyisophthalate derivatives (RO–ip, R = H, Me, Et, Pr and Bn), 1,4-bis(2-methylimidazol-1-yl)benzene (bmib) and Cu(NO3)2·3H2O. Their structures have been confirmed by single crystal X-ray diffraction analyses, IR spectroscopy, TGA and elementary analyses. In 1, μ3-bridged HO–ip links dinuclear Cu(II)–carboxylate units into a 2-D layer, which is further pillared by bmib to generate a two-fold interpenetrating 3-D network. However, μ4-bridged MeO–ip in 2 connects Cu(II) into a 3-D coordination network consisting of tetranuclear Cu(II)–carboxylate units, bmib serves as a void filler by bridging between tetranuclear units. In 3, bis-chelating MeO–ip and bmib link Cu(II) into a 2-D layer, which is packed in an eclipsed pattern. In 4–6, Cu(II) ions are in a distorted square-planar geometry, and carboxylate ligands adopt a bis-monodentate bridging mode and connect the Cu(II) ions into a charge-neutral chain. A pair of such chains in 4 is pillared by bmib to form a pillared-bichain structure, while bmib in 5 and 6 bridges the adjacent chains, resulting in the formation of different 2-D layers. The packing of the 2-D layers in 5 and 6 generates 1-D channels containing guest molecules, which are confirmed by their TGA analyses. The temperature-dependent magnetic analyses show that the dinuclear and tetranuclear Cu(II) units make up the basic magnetic unit of 1 and 2, respectively, and they show an antiferromagnetic interaction.

 Please wait while we load your content...
Please wait while we load your content...