N–H⋯π induced configurational isomerism and the role of temperature in the Z to E isomerization of 2-fluoro-N′-(3-fluorophenyl)benzimidamide†
Abstract
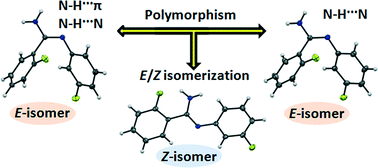
We have investigated the role of N–H⋯π interaction in the formation of Z/E configurational isomers (including dimorphism of the E isomer) of 2-fluoro-N′-(3-fluorophenyl)benzimidamide. The decrease in crystallization temperature by ~20 °C results in the formation of a new isomer (Z isomer). Single crystal X-ray diffraction, thermal characterization (differential scanning calorimetry, hot stage microscopy), FTIR spectroscopy and theoretical calculations have been used to validate the existence of the different conformers in the solid state. The single point energy difference between the E and Z isomer is 21.8 kJ mol−1 and the energy barrier for isomerization is 103.7 kJ mol−1, obtained from theoretical calculations. The difference in thermal energy is similar to the results obtained from theoretical studies, signifying the role of temperature in polymorphism as well as in Z/E isomerization. A comprehensive analysis of the crystal packing and energetic features has been performed based on the molecular conformation and supramolecular packing involving strong N–H⋯N hydrogen bonds, weak C–H⋯N, C–H⋯F, N–H⋯π, and C–H⋯π intermolecular interactions, and π–π stacking to evaluate the role of intermolecular interactions in the solid state. Lattice energies have been calculated using the PIXEL method. Hirshfeld surface fingerprint plots also provide a platform for the evaluation of the contribution of different atom⋯atom contacts to the packing.
- This article is part of the themed collection: Polymorphism

 Please wait while we load your content...
Please wait while we load your content...