A 3D porous supramolecular architecture via π–π assembly of 2D metal–organic frameworks (MOFs): structure-versus-luminescence reversibility and gas adsorption properties†
Abstract
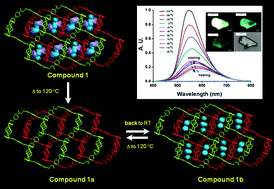
A three-dimensional (3D) porous supramolecular architecture, {[Zn(bdc)(dpds)]·0.62(MeOH)·2H2O}n (1) (bdc2− = dianion of benzenedicarboxylic acid and dpds = 4,4′-dipyridyldisulfide), has been synthesized and structurally characterized. Single-crystal X-ray diffraction analysis reveals that each [Zn2(dpds)2]4+ macrocycle is connected by bdc2− ligands to form a two-dimensional (2D) layered metal–organic framework (MOF). Adjacent layers are further assembled via two interlayer π–π interactions in sandwich-type πpyridyl–πbenzene–πpyridyl and πpyridyl–πpyridyl fashions to afford a 3D porous supramolecular architecture. Controlled heating of the as-synthesized crystal 1 at ~120 °C causes de-solvated species of {[Zn(bdc)(dpds)]}n (1a). The structural determination of the de-solvated compound shows the same structural characterization as that of 1 with the only difference of the nonexistence of solvated MeOH and water molecules. The de-solvated 1a generates the re-hydrated crystal of {[Zn(bdc)(dpds)]·1.1(H2O)}n (1b) upon exposure to water vapor. The water ab-/de-sorption phenomenon by cyclic TG measurement suggests the complete reversibility upon re-/de-hydration between 1a and 1b, associated with reversible temperature-dependent emission properties. Such a reversible switching process of 1 at RT to 120 °C can proceed for at least 20 cycles, implying good thermoluminescence reversibility of 1 during the heating–cooling processes. After removal of the solvent molecules, compound 1a exhibits permanent porosity verified by the N2 sorption isotherm with a Langmuir surface area of 530.0 m2 g−1 and a Brunauer–Emmett–Teller (BET) surface area of 429.4 m2 g−1 and also exhibits significant gas storage capacities of 1.09 wt% for H2 at 77 K and 17 wt% for CO2 at 195 K. Moreover, 1a also displays interesting reversible water, methanol and ethanol vapor ad-/de-sorption behavior correlated with the polarity of the pore surface in 1a to the corresponding adsorbate molecules.

 Please wait while we load your content...
Please wait while we load your content...