Bond-shift isomers: the co-existence of allenic and propargylic phenylnitrile imines†
Abstract
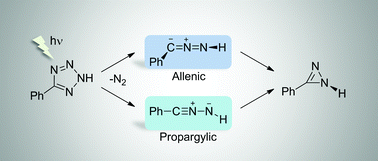
We discovered a 1,3-dipolar species co-existing in two different structures. Photolysis of matrix-isolated 5-phenyltetrazole generates two forms of phenylnitrile imine: propargylic and allenic. They are not resonance structures but correspond to different energy minima, representing bond-shift isomers. These distinct species were characterized spectroscopically and confirmed by calculations up to the CASSCF(14,12) theory level.


 Please wait while we load your content...
Please wait while we load your content...