Four-component reaction leading to highly functionalized sulfoalkoxy carbonyl compounds†
Abstract
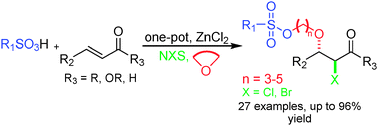
A facile regio- and diastereoselective four-component protocol has been developed involving an α,β-unsaturated carbonyl compound, a cyclic ether, a sulfonic acid and a halogen reagent to access highly anti-α-bromo-β-sulfoalkoxyl carbonyl derivatives. Some of these products have high toxicity against human chronic myeloid leukemia cells.

 Please wait while we load your content...
Please wait while we load your content...