Selective recognition of fluoride anion in water by a copper(ii) center embedded in a hydrophobic cavity†
Abstract
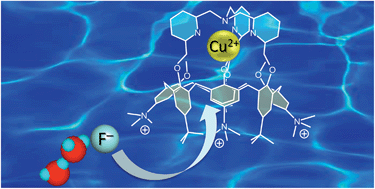
The ability of a water-soluble pentacationic calix[6]arene-based CuII complex to bind anions in water has been explored. Quite remarkably, the complex exhibits strong and selective fluoride binding in the pH range of 6–7. The binding constant at pH 5.9 was evaluated to be 85 000 M−1, which is one of the highest values ever reported for a fluoride probe in water and at this pH. The complex also binds chloride ions, but 1000 times less efficiently. The combination of the calix[6]arene hydrophobic cavity with the CuII complex, presenting its labile site in the endo position, is the reason for the selective recognition process. The single crystal X-ray structure of the organo-soluble parent complex revealed a strong interaction between the coordinated fluoride anion and a hosted CHCl3 solvent molecule. Molecular modeling applying an aqueous environment suggests that a water cluster, [F·H2O·H2O]−, is the species recognized by the host, which provides an appropriate environment for the stabilization of such a hydrated fluoride guest/species.

 Please wait while we load your content...
Please wait while we load your content...