On the factors that control the reactivity of meta-benzynes†
Abstract
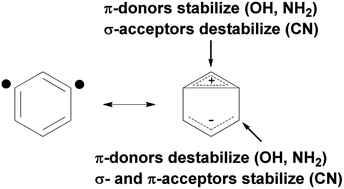
The reactivities of eleven 3,5-didehydropyridinium and six 2,4-didehydropyridinium cations toward cyclohexane were examined in the gas phase by using Fourier-transform ion cyclotron resonance (FT-ICR) mass spectrometry as well as high-level quantum chemical calculations. The results unequivocally demonstrate that the reactivity of meta-benzyne analogs can be “tuned” from more radical-like to less radical-like by changing the type and position of substituents. For example, σ-acceptor substituents at the 4-position and π-donor substituents at the 2-position in 3,5-didehydropyridinium cations partially decouple the biradical electrons, which results in lower energy transition states, and faster radical reactions. In contrast, σ-acceptors at the 2-position and π-donors at the 4-position in 3,5-didehydropyridinium cations cause stronger coupling between the biradical electrons, which results in lower radical reactivity. Three main factors are found to control the reactivity of these biradicals: (1) the energy required to distort the minimum energy dehydrocarbon atom separation to the separation of the transition state, (2) the S–T splitting at the separation of the transition state, and (3) the electron affinity at the separation of the transition state.

 Please wait while we load your content...
Please wait while we load your content...