Aqueous organocatalyzed aldol reaction of glyoxylic acid for the enantioselective synthesis of α-hydroxy-γ-keto acids†
Abstract
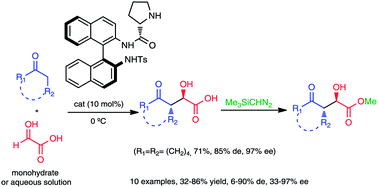
N-Tosyl-(Sa)-binam-L-prolinamide is an efficient catalyst for the aqueous aldol reaction, between glyoxylic acid, as monohydrate or aqueous solution, and ketones. This reaction led to the formation of chiral α-hydroxy-γ-keto carboxylic acids in high levels of diastereo- and enantioselectivities achieving mainly anti aldol products.

 Please wait while we load your content...
Please wait while we load your content...