A block copolymer-stabilized co-precipitation approach to magnetic iron oxide nanoparticles for potential use as MRI contrast agents†
Abstract
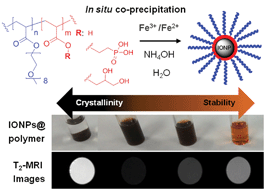
A library of magnetic nanoparticles was generated using in situ co-precipitation of ferrous (Fe2+) and ferric (Fe3+) ions from aqueous solutions in the presence of functional block copolymers. Three different iron oxide anchoring groups, viz., phosphonic acid, carboxylic acid or glycerol were incorporated into well-defined diblock copolymers of poly(oligoethylene glycol acrylate) employed to stabilize the iron oxide nanoparticles. The [copolymer] : [Fe] ratio was varied to wield control over nanoparticle diameters within the range of 7–20 nm. The relationship between colloidal stability and nanoparticle crystallinity was investigated using dynamic light scattering, transmission electron microscopy and X-ray diffraction measurements. The amount of polymer employed during the co-precipitation proved critical in governing crystallinity and colloidal stability. We report a correlation between the polymer grafting density and the chemical structure of the anchoring group. Finally, the transverse relaxivity of the iron oxide nanoparticles in water, was investigated using a 9.4T magnetic resonance imaging scanner yielding values varying from 70 to 370 mM−1 s−1.
- This article is part of the themed collection: Polymer Chemistry Lectureship Winners

 Please wait while we load your content...
Please wait while we load your content...