Photoswitchable fluorescent diheteroarylethenes: substituent effects on photochromic and solvatochromic properties†
Abstract
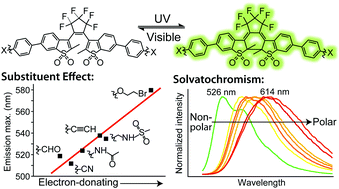
Photoswitchable fluorescent diheteroarylethenes are promising candidates for applications in super-resolution molecular localization fluorescence microscopy thanks to their high quantum yields and fatigue-resistant photoswitching characteristics. We have studied the effect of varying substituents on the photophysical properties of six sulfone derivatives of diheteroarylethenes, which display fluorescence in one (closed form) of two thermally stable photochromic states. Electron-donating substituents displace the absorption and emission spectra towards the red without substantially affecting the fluorescence quantum yields. Furthermore, ethoxybromo, a very electron-donating substituent, stabilizes the excited state of the closed isomer to the extent of almost entirely inhibiting its cycloreversion. Multi-parameter Hammett correlations indicate a relationship between the emission maxima and electron-donating character, providing a useful tool in the design of future photochromic molecules. Most of the synthesized compounds exhibit small bathochromic shifts and shorter fluorescence lifetimes with an increase in solvent polarity. However, the ethoxybromo-substituted fluorescent photochrome is unique in its strong solvatochromic behaviour, constituting a photoactivatable (photochromic), fluorescent and highly solvatochromic small organic compound. The Catalán formalism identified solvent dipolarity as the principal basis of the solvatochromism, reflecting the highly polarized nature of this molecule.

 Please wait while we load your content...
Please wait while we load your content...