Folded alkyl chains in water-soluble capsules and cavitands†
Abstract
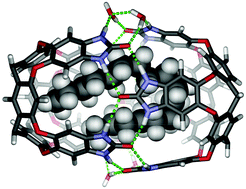
A deep cavitand with ionic “feet” dimerizes around hydrophobic compounds in D2O. Longer n-alkane guests, C14–C18, are encapsulated in contorted conformations and NMR is used to deduce their shapes. Competition experiments establish the driving forces involved and how they compensate for the steric clashes in the folded structures of the encapsulated alkanes. Bolaamphiphiles instead prefer to bind in the monomeric cavitand with conformations that bury the methylenes but expose the polar head groups to solvent.
- This article is part of the themed collection: Supramolecular Chemistry in Water

 Please wait while we load your content...
Please wait while we load your content...