In situ oxygenous functionalization of a graphite electrode for enhanced affinity towards charged species and a reduced graphene oxide mediator†
Abstract
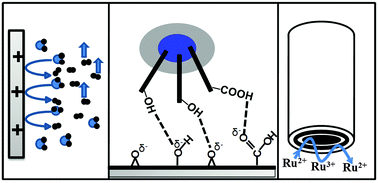
The weak affinity of bare carbon based electrodes for biological molecules or charged species is a major drawback for their direct application in analytical electrochemistry. We observed that the surfaces of graphite rods and glassy carbon (GC) ring electrodes can be modified by oxygenated functional groups through controlled electrochemical oxidation in aqueous media. Study of their cyclic voltammetry, surface conductivity, X-ray photoelectron spectroscopy (XPS), Raman spectroscopy, infrared spectroscopy (IR) and powder X-ray diffraction (PXRD) spectroscopy confirmed the chemical change. In the electrodynamics study, the modified GC ring in a rotating ring-disk electrode (RRDE) assembly showed better electron transfer efficiency than the virgin electrode when evaluated using a Ru(bpy)32+/3+ couple. In comparison to the virgin electrode, the modified graphite rod exhibited better affinity toward polyvinylpyrrolidone protected reduced graphene oxide (PVP-rGO) attached to glucose oxidase enzymes (GOx), and showed the direct attachment of mediators to the oxygenated electrode surface. These observations imply that a small oxygenated layer on the carbon electrode surface can significantly increase its activity. The present evidence indicates the possibility for the oxygenous functionalization of carbon based electrodes for applications in areas like electrokinetics studies and biosensing, where strong analyte–electrode interactions are useful.
- This article is part of the themed collection: Advanced Complex Inorganic Nanomaterials

 Please wait while we load your content...
Please wait while we load your content...