Aqueous phase reforming of glycerol over Re-promoted Pt and Rh catalysts†
Abstract
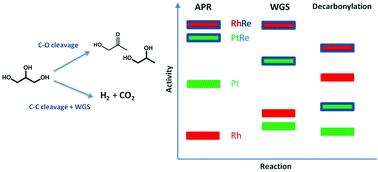
The potential of Re promotion for carbon-supported Pt and Rh nanoparticles was investigated for aqueous phase reforming (APR) of glycerol as a model compound for biobased feedstock. Upon alloying with Re, the overall conversion rate of glycerol was substantially increased for both metal catalysts. Whereas Pt/C is more active than Rh/C in glycerol APR, the RhRe/C catalyst outperforms PtRe/C. The overall APR catalytic performance strongly correlates with the activity trend for the gas-phase water–gas shift (WGS) reaction. RhRe/C exhibited the highest activity in APR and WGS reactions. A very strong synergy between Rh and Re and Pt and Re is observed in the model WGS reaction. The role of Re in the bimetallic catalysts is to facilitate water dissociation, effectively increasing the WGS activity. During APR, this results in lower steady-state CO coverage and increased glycerol conversion rates. In terms of selectivity, the yield of renewable hydrogen is increased. The use of Re as a promoter also results in significant changes in the product selectivities during glycerol APR. Although gas-phase acetaldehyde decomposition measurements evidenced that alloying with Re increased C–C bond cleavage activities of Pt/C and Rh/C, the increased acidity due to acidic hydroxyl groups bound to Re resulted in a more substantial increase of dehydration reactions. Whereas Rh/C is more selective for formation of products involving C–C bond cleavage than Pt/C, the product mixtures of their alloys with Re reflect a much increased ratio of C–O vs. C–C bond cleavage reaction rates.
- This article is part of the themed collection: Conversion of biomass with heterogeneous catalysts

 Please wait while we load your content...
Please wait while we load your content...