Femtosecond spectroscopy of the dithiolate Cu(ii) and Ni(ii) complexes
Abstract
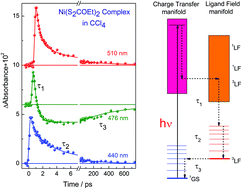
Femtosecond spectroscopy was applied to study the ultrafast dynamics for the excited states of dithiolate Cu(II) and Ni(II) complexes. The detailed information on the initial steps after the absorption of a photon by the metal complexes is of fundamental importance to understand the mechanism of photochemical reactions. The fast processes for the dithiolate complexes have hardly been studied. In this review the spectra of transients and their lifetimes will be presented. For example, the xanthogenate Ni(S2COEt)2 complex in acetonitrile and CCl4 after the pulse of the second harmonic (100 fs, 400 nm) of a Ti:S laser moves to the excited 1LMCT state which decays in 0.76 ps to the excited 3LF state. In 6.8 ps the 3LF state undergoes vibrational cooling and then it slowly decays in 550 ps to the ground state. However, for many dithiolate complexes the kinetic curves can be well treated in a two-exponential approximation. A short time (less than 1 ps) may include several processes (relaxation of the Franck–Condon state, redistribution of vibrational energy (IVR), internal conversion (IC) and intersystem crossing (ISC)). A long time (a few picoseconds) usually reflects the vibrational cooling of the ground state. The quantum yields of the dithiolate and dithiolene complex disappearance in halogen containing solvents have a strong dependence on the wavelength of irradiation. It is very likely that electron transfer to the acceptor becomes effective when the electron in the excited complex moves to antibonding ligand orbitals localized at the periphery of the complex close to the acceptor molecule (halogenated solvent).
- This article is part of the themed collection: Spectroscopy of Inorganic Excited States

 Please wait while we load your content...
Please wait while we load your content...