An examination of the intrinsic activity and stability of various solid acids during the catalytic decarboxylation of γ-valerolactone
Abstract
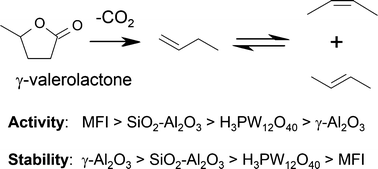
Rates of γ-valerolactone (GVL) decarboxylation were measured in the gas phase under anhydrous conditions from 523–723 K over a series of solid acids including amorphous silica alumina, MFI zeolites, supported phosphotungstic acid, and γ-Al2O3. Through consideration of decarboxylation rates obtained under differential conditions, we examine the roles of Brønsted and Lewis acidity, deprotonation energy, and catalyst morphology in defining the intrinsic activity and stability of each material. In aluminosilicates, Brønsted sites associated with framework aluminum appear to contribute the majority of decarboxylation activity. Of the aluminosilicates tested, Brønsted sites in MFI are more intrinsically active than analogous sites in ASA; however, zeolite micropores hinder GVL diffusion and lead to mass transfer limitations at high temperatures. Relative to bridging hydroxyls, coordinatively unsaturated aluminum sites are substantially less active and do not contribute significantly to decarboxylation rates in materials having both framework and extraframework aluminum. Decarboxylation barriers scale with the deprotonation energy of Brønsted acid sites; however, lower deprotonation energies do not necessarily imply higher intrinsic activity in GVL decarboxylation. At 623 K, catalyst stability is highest in materials having large pore dimensions, Lewis sites as primary catalytic centers, and Brønsted sites with relatively high deprotonation energies.
- This article is part of the themed collection: Sustainable catalytic conversions of renewable substrates

 Please wait while we load your content...
Please wait while we load your content...