First and second deprotonation of H2SO4 on wet hydroxylated (0001) α-quartz†
Abstract
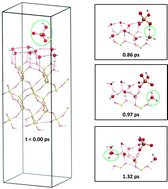
We present an ab initio molecular dynamics study of deprotonation of sulfuric acid on wet quartz, a topic of atmospheric interest. The process is preferred, with 65% of our trajectories at 250 K showing deprotonation. The time distribution of the deprotonation events shows an exponential behavior and predicts an average deprotonation time of a few picoseconds. The process is exoergic, with most of the temperature increase being due to formation of hydrogen bonds prior to deprotonation. In agreement with existing studies of H2SO4 in water clusters, in liquid water, and at the air–water interface, the main determinant of deprotonation is the degree of solvation of H2SO4 by neighboring water molecules. However, we find that if both hydrogens of H2SO4 are simultaneously donated to water oxygens, deprotonation is disfavored. Predicted spectroscopic signatures showing the presence of solvated hydronium and bisulfate are presented. Increasing the temperature up to 330 K accelerates the process but does not change the main features of the deprotonation mechanisms or the spectroscopic signatures. The second deprotonation of H2SO4, studied only at 250 K, occurs provided there is sufficient solvation of the bisulfate by additional water molecules. In comparison to HCl deprotonation on the identical surface examined in our previous work, the first deprotonation of H2SO4 occurs more readily and releases more energy.

 Please wait while we load your content...
Please wait while we load your content...