Electron induced single strand break and cyclization: a DFT study on the radiosensitization mechanism of the nucleotide of 8-bromoguanine
Abstract
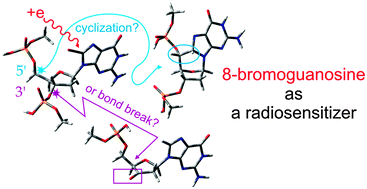
Cleavage of the O–P bond in 8-bromo-2′-deoxyguanosine-3′,5′-diphosphate (BrdGDP), considered as a model of single strand break (SSB) in labelled double-stranded DNA (ds DNA), is investigated at the B3LYP/6-31++G(d,p) level. The thermodynamic and kinetic characteristics of the formation of SSB are compared to those related to the 5′,8-cycloguanosine lesion. The first reaction step, common to both damage types, which is the formation of the reactive guanyl radical, proceeds with a barrier-free or low-barrier release of the bromide anion. The guanyl radical is then stabilized by hydrogen atom transfer from the C3′ or C5′ sites of the 2′-deoxyribose moiety to its C8 center. The C3′ path, via the O–P bond cleavage, leads to a ketone derivative (the SSB model), while the C5′ path is more likely to yield 5′,8-cycloguanosine.

 Please wait while we load your content...
Please wait while we load your content...