Non-covalent interactions of the carcinogen (+)-anti-BPDE with exon 1 of the human K-ras proto-oncogene†
Abstract
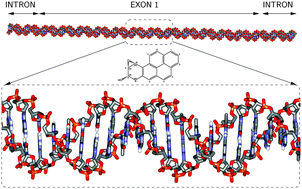
Investigating the complementary, but different, effects of physical (non-covalent) and chemical (covalent) mutagen–DNA and carcinogen–DNA interactions is important for understanding possible mechanisms of development and prevention of mutagenesis and carcinogenesis. A highly mutagenic and carcinogenic metabolite of the polycyclic aromatic hydrocarbon benzo[α]pyrene, namely (+)-anti-BPDE, is known to undergo both physical and chemical complexation with DNA. Previous studies of BPDE–DNA complex formation have focused on processes that require substantial structural reorganization, such as intercalation, and consequently relatively long time scales. However, some initial processes which occur within shorter time scales, such as external non-covalent binding, and which do not require major DNA structural reorganization have not been thoroughly investigated. A detailed computational study of such initial BPDE–DNA interactions is needed to elucidate the temporal and structural origins of the major covalent adduct, a promutagenic, which is known to exist in an external (+)-trans-anti-BPDE-N2-dGuanosine configuration. Accordingly, the initial stages of external non-covalent BPDE–DNA binding are studied in this work as well as their relationship to subsequent formation of the major, also external, covalent adduct. To study mechanisms that occur prior to extensive DNA structural reorganization, we present a first and detailed codon by codon computational study of the non-covalent interactions of (+)-anti-BPDE with DNA. In particular, due to its relevance to carcinogenesis, the interaction of (+)-anti-BPDE with exon 1 of the human K-ras gene has been studied. External solvent-exposed non-covalent binding sites have been found which may be precursors of the major external trans adduct and, importantly, are located in codons 12 and 13 of the K-ras gene which are known to be key mutation hotspots. In addition, our study explains and correctly predicts preferential (+)-anti-BPDE binding at minor groove guanosines. A subtle combination of van der Waals and hydrogen bonding interactions has been found to be a primary factor in preferentially positioning (+)-anti-BPDE toward the 5′ position of a guanosine's strand, consistent with proton NMR observations for the major trans adduct, and at 5′-TGG-3′ sequences which are known to yield high binding probability.

 Please wait while we load your content...
Please wait while we load your content...