Structural, spectroscopic and theoretical studies of a vapochromic platinum(ii) terpyridyl complex†
Abstract
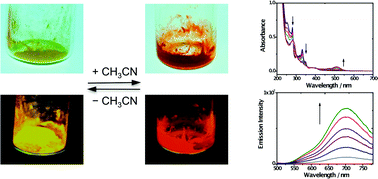
The vapochromism of the platinum(II) terpyridyl complex [Pt(tpy)Cl](PF6) (1, tpy = 2,2′:6′,2′′-terpyridine) was investigated. Complex 1 was found to exist in two forms. The yellow form of 1 turned to red by exposing the solid to either vapor or solution of acetonitrile, accompanied by changes in luminescence spectroscopy. This process could be reversed upon the loss of acetonitrile. Complex 1 was also demonstrated to show aggregation in a diethyl ether–acetonitrile system through Pt⋯Pt and terpyridyl π–π interactions to afford the red form of 1. Crystals of the red and yellow forms of 1 were analyzed. The red form of 1 crystallizes in the orthorhombic space group Pnma with a co-crystallized acetonitrile solvent molecule and thus is defined as 1-MeCN, while the yellow form is found to have the previously reported non-solvated crystal structure of 1. In 1-MeCN, the square planar [Pt(tpy)Cl]+ monocations stack to give an extended chain-like array of Pt atoms with a short Pt⋯Pt distance of 3.362 Å, while in 1, there are two alternating Pt⋯Pt distances (4.032 and 3.340 Å). Time-dependent density functional theory (TDDFT) calculations demonstrated that Pt⋯Pt and terpyridyl π–π interactions play important roles in electronic absorption features of the [Pt(tpy)Cl]+ system. Compared to dimers, the stack of three molecules of 1 with a short Pt⋯Pt distance considerably lowers the transition energy of metal–metal-to-ligand charge transfer (MMLCT), which causes a dramatic red shift in UV-vis spectroscopy.

 Please wait while we load your content...
Please wait while we load your content...