The role of carbonate as a catalyst of Fenton-like reactions in AOP processes: CO3˙− as the active intermediate†
Abstract
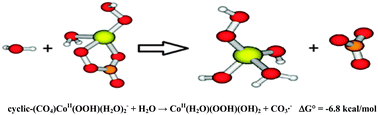
Kinetic and DFT results for the carbonate catalysed Co(H2O)62+ + H2O2 Fenton-like reaction suggest a mechanism involving the formation of a cyclic transient, cyclic-(CO4)CoII(OOH)(H2O)2− that decomposes into CoII(H2O)(OOH)(OH)2 + CO3˙−, i.e. no OH˙ radicals are involved. Plausible biological implications are pointed out.

 Please wait while we load your content...
Please wait while we load your content...