Stereoselective construction of a key hydroindole precursor of epidithiodiketopiperazine (ETP) natural products†
Abstract
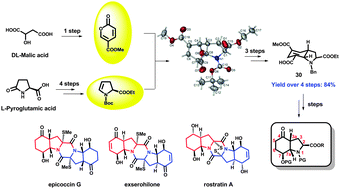
An asymmetric synthetic strategy for constructing the divergent-synthesis monomer of epidithiodiketopiperazine (ETP) natural products has been successfully developed. The functionalized 2,3,3a,4,7,7a-hexahydroindole scaffold was constructed by a diastereoselective inverse electron-demand Diels–Alder (IEDDA) reaction.

 Please wait while we load your content...
Please wait while we load your content...