Rapid synthesis and electrical transition in p-type delafossite CuAlO2
Abstract
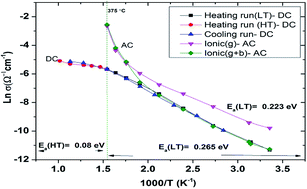
Highly polycrystalline and pure delafossite phase CuAlO2 powder has been synthesised within a short annealing period, shorter than most conventional processes. This is an improvement over the conventional synthesis procedures. The conventional synthesis procedure has seen CuAlO2 only formed at high annealing temperatures ≥1100 °C over long annealing time, some as long as 96 hours. In the current process, a pure phase devoid of impurities has been obtained at a reduced calcination time of 1.5 hours in an argon atmosphere at a temperature of 1150 °C. This was confirmed by XRD and SEM/EDX. High temperature DC/AC electrical measurements show a change in the conduction mechanism from mixed conductivity (ionic + p-type) in the temperature range of 375 ≥ T ≥ 25 °C to intrinsic type behavior above 375 °C. The activation energies for these two regimes are 0.27 eV and 0.08 eV respectively. This change from mixed to p-type conductivity is confirmed by spectral analysis also. Spectral analysis using the power law also revealed that conduction is of long range hopping. The use of platinum as a contact electrode at elevated temperatures has a detrimental effect on the electrical properties since it encourages the formation of CuAl2O4 at the interface due to the formation of a more stable Cu–Pt alloy by virtue of the chemical reaction 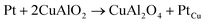 .
.

 Please wait while we load your content...
Please wait while we load your content...