Mechanism of the reduction and energy transfer between Eu2+ and Eu3+ in Eu-doped CaAl2Si2O8 materials prepared in air†
Abstract
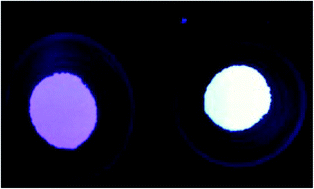
Eu2+ doped CaAl2Si2O8 materials prepared under a reducing atmosphere show a white-bluish luminescence under UV excitation. Eu3+ cations naturally give rise to line emission spectra for which the colour of emissions is between orange and red. To achieve a warm color or to change the CIE(x, y) position of the Ca1−xEuxAl2Si2O8 phosphors, we decided to investigate the synthesis of these compounds under air conditions to favour the co-existence of Eu2+ and Eu3+ cations and investigate the mechanism of the “abnormal” reduction from Eu3+ to Eu2+ and the energy transfer from Eu2+ to Eu3+ in the “co-doped” CaAl2Si2O8 host lattice. The results indicate that both Eu2+ and Eu3+ co-exist in the aluminosilicates, even when synthesized under air conditions, due to the substitution of Eu3+ cations for alkaline earth metal (Ca2+) triggering the formation of vacancies that play the role of electron donors towards V//Ca defects. The higher the concentration of V//Ca, the easier the reduction of Eu3+ into Eu2+. Consequently, the “co-doping” with higher state chemical elements (e.g. Zr4+) may help a lot in increasing the concentration of Eu2+ cations at the expense of Eu3+. We will take advantage of this observation in the future for sure to help prepare materials.

 Please wait while we load your content...
Please wait while we load your content...