Barium peroxide nanoparticles: synthesis, characterization and their use for actuating the luminol chemiluminescence
Abstract
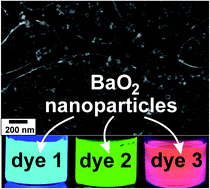
Barium peroxide (BaO2) nanoparticles are prepared via microemulsion techniques, using concentrated hydrogen peroxide (perhydrol, 30%) as a polar micelle phase. The as-prepared BaO2 nanoparticles are characterized by SEM, STEM, XRD, and FT-IR to validate the particle size (40 nm in diameter) and phase purity (e.g., the absence of BaO, Ba(OH)2, and BaCO3). BaO2 nanoparticles are strong oxidizing agents due to in situ release of O2 as is proven by de-N-acetylation of Ampliflu Red and the strong red fluorescence of resorufin. Moreover, they can be used for luminol-driven chemiluminescence detection of Fe2+/Fe3+ (<0.05 mmol) as an alternative to H2O2. In comparison with conventional aqueous H2O2, BaO2 nanoparticles show a high storage stability (without autocatalytic decomposition) and a 5-times more intense chemiluminescence with luminol. Via fluorescence transfer, the chemiluminescence can also be shifted from blue (luminol) to green (fluorescein), which is at optimal eye sensitivity and therefore facilitates naked-eye detection. The newly prepared BaO2 nanoparticles as an active peroxide and strong oxidizing agent can be of potential interest for organic synthesis, CO2 adsorption, disinfection, wastewater treatment as well as for luminol-driven chemiluminescence detection of iron in analytical chemistry and criminology.

 Please wait while we load your content...
Please wait while we load your content...