5,5′-Bis-(alkylpyridinyl)-2,2′-bithiophenes: synthesis, liquid crystalline behaviour and charge transport†
Abstract
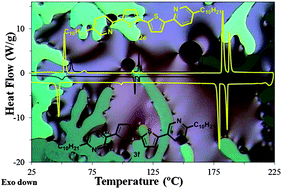
Liquid crystalline materials with 5,5′-bis-(alkylpyridinyl)-2,2′-bithiophene structures with different positions of the nitrogen atoms in the aromatic core were synthesized, characterized by differential scanning calorimetry, polarized optical microscopy and, in some cases, by synchrotron X-ray diffraction analysis and single crystal X-ray analysis. The molecular planarity and liquid crystalline (LC) behaviour of these materials are strongly influenced by the location of the nitrogens in the aromatic core, and LC behaviour is more complex than the mesomorphic behaviour of the known structurally related 5,5′-bis-(alkylphenyl)-2,2′-bithiophene LCs. Single crystal X-ray structural analysis of 5,5′-bis-(5-alkylpyridin-2-yl)-2,2′-bithiophenes 2 reveals that the aromatic core is nearly planar with s-cis conformation of the thiophene and pyridine rings and s-trans conformation of the thiophene rings of the central 2,2′-bithiophene unit. The length of the alkyl chains has a pronounced effect on the molecular planarity and the packing motifs. A preliminary study of the charge-transporting properties of 5,5′-bis-(5-alkylpyridin-2-yl)-2,2′-bithiophenes 2d and 2e with n-nonyl and n-decyl alkyl chains, respectively, by the time-of-flight technique shows that the hole mobility is temperature independent, electric field dependent and with a magnitude of ∼1.5 × 10−4 cm2 V−1 s−1 in the unidentified S2/Cr2 phase.

 Please wait while we load your content...
Please wait while we load your content...