Nanoscale in situ detection of nucleation and growth of Li electrodeposition at various current densities†
Abstract
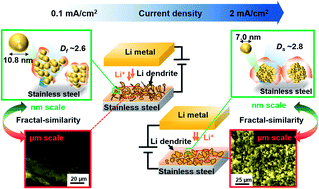
Li metal batteries can store at least ten times more energy than currently existing Li-ion batteries. However, during routine charging and discharging, Li dendrites grow on the Li metal electrode, which can lead to capacity loss by the consumption of Li salt at the surface of the Li dendrites, and be a safety hazard resulting from the potential for short-circuits. Although past efforts have provided useful information about the morphology and surface area of Li dendrite formation at the microscale, a nanoscale understanding of nucleation and growth of Li nanoparticle electrodeposition is still elusive. In this study, using a new electrochemical cell for transmission mode grazing incidence small angle X-ray scattering, we obtained, for the first time, the primary nucleus size of Li nanoparticles, their size evolution and their fractal structures at various current densities and in real-time. The measured average radius of gyration, Rg, at current densities of 0.1, 0.5, and 2.0 mA cm−2 is 5.4 ± 0.4, 4.5 ± 0.3, and 3.5 ± 0.3 nm, respectively. This variation in size with current density is noteworthy when recognizing that the surface area-to-volume ratio of the Li nanoparticles is 3.7 times higher at 2.0 mA cm−2 than at 0.1 mA cm−2. We also compared a hierarchical fractal structure of Li particles from the nanometer to micrometer scale. Our findings illuminate the role of overpotential in the reactive surface area of Li dendrites at the nanoscale, and provide a novel research platform for suppressing Li dendrite formation in Li metal battery systems.


 Please wait while we load your content...
Please wait while we load your content...