Crumpled reduced graphene oxide by flame-induced reduction of graphite oxide for supercapacitive energy storage†
Abstract
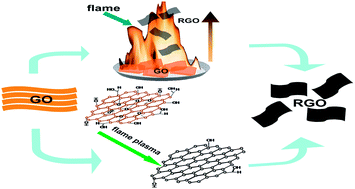
A novel flame-induced synthesis approach was developed to prepare crumpled reduced graphene oxide (rGO) from graphite oxide (GO) powder with the assist of flammable polar solvents such as methanol, ethanol and acetone under ordinary conditions. The as-prepared methanol–rGO, ethanol–rGO and acetone–rGO exhibit a high surface area of 421, 500 and 384 m2 g−1, respectively. The method is highly attractive for the mass production of graphene due to its simplicity, high efficiency, energy saving property and cost-effectiveness. The thermal exfoliation and reduction of GO powder, as well as the morphology and surface chemistry of resulting rGO, were related to the volatility, infiltration and combustion heat of the solvents. The electrochemical properties of the rGO samples were further evaluated in a 6 M KOH aqueous electrolyte. In a three-electrode setup, the corresponding specific capacitance of methanol–rGO, ethanol–rGO and acetone–rGO was calculated to be 260, 221 and 200 F g−1 at a current density of 0.1 A g−1, respectively. Moreover, the flame-reduced methanol–rGO exhibited a maximum energy density of 68.85 W h kg−1 as tested in a two-electrode system. The excellent supercapacitive performance of the methanol–rGO and ethanol–rGO materials is attributable to the combination of high surface area, residual oxygen containing groups and wrinkled morphologies.

 Please wait while we load your content...
Please wait while we load your content...