Room-temperature synthesis of Pd/C cathode catalysts with superior performance for direct methanol fuel cells†
Abstract
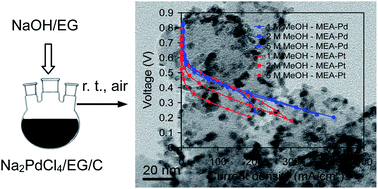
Palladium nanoparticles supported on carbon black (Pd/C) have been successfully synthesized in a dispersion of sodium hydroxide, ethylene glycol, and carbon black (NaOH/EG/C) at room temperature without any protection with an inert gas atmosphere. Carbon black and EG dehydrated by NaOH (0.02 M) synergistically reduce Na2PdCl4 to metallic Pd at room temperature with a conversion efficiency of >99%. The capability of carbon black to provide the reducing function in the synthesis was verified by Fourier transform infrared (FTIR) spectroscopic data. When the NaOH concentration is <0.01 M, the reduction reaction is far from completion due to the very low degree of dehydration of EG. When the NaOH concentration is >0.05 M, the reduction reaction rate is slow owing to strong complexation between Pd2+ and OH− and the oxidative etching of the formed Pd nanoparticles by air and OH−. The synthesized Pd/C samples with Pd loadings of 20 and 40 wt% have Pd particle sizes of, respectively, 3.35 ± 0.73 nm and 3.86 ± 0.74 nm, uniformly loaded onto the carbon black support. Compared to commercial Pt/C (40 wt%) catalysts, the Pd/C (40 wt%) catalyst synthesized here shows superior methanol tolerance and performance as a cathode catalyst in a direct methanol fuel cell (DMFC).

 Please wait while we load your content...
Please wait while we load your content...