Chemical transformations of nanomaterials for energy applications
Abstract
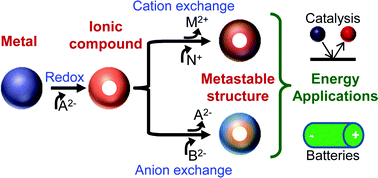
Chemical Transformations of Nanomaterials (CTNMs) is a powerful method to modify as-synthesized nanomaterials into forms with novel composition and structure. CTNMs allows access to nano-based materials that are difficult to synthesize by conventional means, including metastable materials through partial transformations. These new nanomaterial forms can have property and stability advantages when used for energy applications. This short review highlights recent research progress in chemical transformations of non-metallic nanomaterials as a means to create useful energy materials, such as metastable structures, with a focus on fuel cell catalysts and battery technologies. In addition to discussing progress with chemically transformed materials, this review briefly highlights several metastable materials that have been used for energy applications but were synthesized through conventional means. Synthetic protocols of CTNMs could be used to produce these metastable energy materials, which may result in novel properties. Finally, the review concludes with a forward looking perspective of metastable materials that have yet to be synthesized, but are good candidates for catalysis/batteries as metastable materials. These proposed systems could yield next generation catalysts/battery materials.
- This article is part of the themed collection: Emerging Investigators

 Please wait while we load your content...
Please wait while we load your content...