A theoretical approach to evaluate the rate capability of Li-ion battery cathode materials†
Abstract
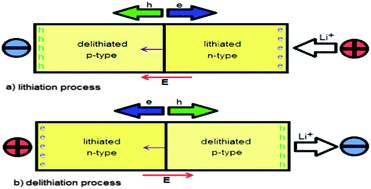
Charge–discharge rate capability is one of the most important properties of cathode materials for lithium batteries, in particular when envisaging high power density applications such as automotive applications. Efforts to modify rate have been carried out by carbon coating and decreasing particle size in order to modify electronic and ionic conductivity. However, this approach cannot justify all experimental data reported in the literature. Here, we investigated the rate capability of cathode materials by considering their density of states (DOS) calculated by several density functional theory (DFT) methods, in both the lithiated and the delithiated case. We suggested that these structures could be interpreted as n- or p-type semiconductors, depending on the DOS configuration. On this basis, if the lithiated structure acted as an n-type and the delithiated one as a p-type semiconductor, the resulting cathode will only be capable of achieving a “low rate”. If the opposite situation happened, the cathode would sustain high current rates. Li2FeSiO4, LiFePO4, LiFeBO3 and LiFeSO4F were found to belong to the former class, whereas LiCoO2, LiFeO2 and LiMn2O4 were assigned to the latter one. On the basis of the proposed model, we suggested some general strategies related to the synthetic approach to improve cathode rate capability.

 Please wait while we load your content...
Please wait while we load your content...