Isolation of the key intermediates of base-promoted borylene–carbonyl coupling reactions†
Abstract
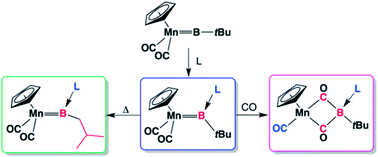
The terminal alkylborylene complex [(η5–C5H5)(OC)2Mn![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) BtBu] (1) reacts with trimethylphosphine and CO to form a borylene–carbonyl coupling product [(η5–C5H5)(OC)Mn{κ2–C,C′–C(O)B(tBu)(PMe3)C(O)}] (3). This reactivity can be extended to nitrogen- and carbon-based ligands to form similar complexes [(η5–C5H5)(OC)Mn{κ2–C,C′–C(O)B(tBu)(L)C(O)}] (L = DMAP, 7, and IMe, 8). The key intermediates of these reactions were found to be neutral, Lewis base-stabilized borylenes of the form [(η5–C5H5)(OC)2Mn
BtBu] (1) reacts with trimethylphosphine and CO to form a borylene–carbonyl coupling product [(η5–C5H5)(OC)Mn{κ2–C,C′–C(O)B(tBu)(PMe3)C(O)}] (3). This reactivity can be extended to nitrogen- and carbon-based ligands to form similar complexes [(η5–C5H5)(OC)Mn{κ2–C,C′–C(O)B(tBu)(L)C(O)}] (L = DMAP, 7, and IMe, 8). The key intermediates of these reactions were found to be neutral, Lewis base-stabilized borylenes of the form [(η5–C5H5)(OC)2Mn![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) B(tBu)(L)] (L = 4-dimethylaminopyridine (DMAP), 4, 3,5-lutidine, 5 and :C[N(Me)CH]2 (IMe), 6, which have been isolated and fully characterized. They can be synthesized from simple addition of the base to the parent borylene 1. The coordination of the base to the boron center seems to facilitate a rearrangement of the t-butyl borylene substituent.
B(tBu)(L)] (L = 4-dimethylaminopyridine (DMAP), 4, 3,5-lutidine, 5 and :C[N(Me)CH]2 (IMe), 6, which have been isolated and fully characterized. They can be synthesized from simple addition of the base to the parent borylene 1. The coordination of the base to the boron center seems to facilitate a rearrangement of the t-butyl borylene substituent.

 Please wait while we load your content...
Please wait while we load your content...