Affinity-tunable specific recognition of glycoproteins via boronate affinity-based controllable oriented surface imprinting†
Abstract
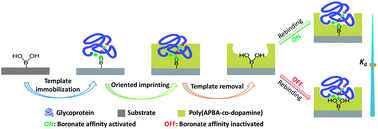
The molecular imprinting of proteins is of great importance but remains a challenge. Particularly, efficient, universal and facile approaches for protein imprinting are limited. Here we report a new general approach, boronate affinity-based controllable oriented surface imprinting, for the efficient and facile imprinting of glycoproteins. A glycoprotein template was first covalently anchored onto the surface of a boronic acid-functionalized substrate by boronate affinity binding. The substrate surface was then deposited with a thickness-controllable imprinting coating generated by in-water self-copolymerization of dopamine and m-aminophenylboronic acid (APBA). After removal of the template with an acidic solution, 3D cavities complementary to the molecular shape of the template were formed in the imprinting layer. The imprinting layer was hydrophilic and showed limited residual boronic acid, thus non-specific binding was avoided. The approach has significant advantages, including high specificity, high imprinting efficiency, and widely applicable substrates (from 2D to 3D, from regular size to nanoscale). Uniquely, the prepared molecularly imprinted polymers can rebind the templates in dual modes: a high affinity mode (boronate affinity interaction is on) and a low affinity mode (boronate affinity interaction is off), and the overall binding strength can be tuned by adjusting the surrounding pH. Such an affinity-tunable dual-mode binding mechanism enables the binding strength to be adjusted while keeping the specificity, which allows for wider applications, and also sheds new light on the role of affinity-determining factors in molecular imprinting.

 Please wait while we load your content...
Please wait while we load your content...