Do spin state and spin density affect hydrogen atom transfer reactivity?
Abstract
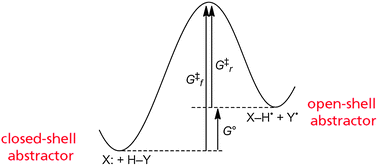
The prevalence of hydrogen atom transfer (HAT) reactions in chemical and biological systems has prompted much interest in establishing and understanding the underlying factors that enable this reactivity. Arguments have been advanced that the electronic spin state of the abstractor and/or the spin-density at the abstracting atom are critical for HAT reactivity. This is consistent with the intuition derived from introductory organic chemistry courses. Herein we present an alternative view on the role of spin state and spin density in HAT reactions. After a brief introduction, the second section introduces a new and simple fundamental kinetic analysis, which shows that unpaired spin cannot be the dominant effect. The third section examines published computational studies of HAT reactions, which indicates that the spin state affects these reactions indirectly, primarily via changes in driving force. The essay concludes with a broader view of HAT reactivity, including indirect effects of spin and other properties. It is suggested that some of the controversy in this area may arise from the diversity of HAT reactions and their overlap with proton-coupled electron transfer (PCET) reactions.

 Please wait while we load your content...
Please wait while we load your content...