Morphologic control of Sb-rich Sb2Se3 to adjust its catalytic hydrogenation properties for p-nitrophenol†
Abstract
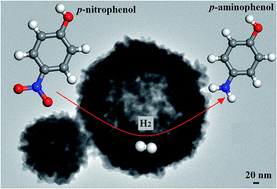
Sb-rich Sb2Se3 with hollow spherical morphologies and Se-rich Sb2Se3 with rod-like shapes were prepared by a hydrothermal method using sodium borohydride as the reductant. It was found that the material with high Sb content and microsphere-like morphology exhibits efficient catalytic performance for the hydrogenation of p-nitrophenol. A systematic study on the effect of hydrothermal reaction time on the structure and morphology of Sb-rich Sb2Se3 showed that the products are mainly composed of rod-like and hollow sphere-like Sb2Se3 under the different conditions; the microspherical particles are mainly composed of Sb and Sb2Se3, while the rod-like particles are made of Se-rich Sb2Se3. The longer hydrothermal reaction duration with an Sb/Se atomic ratio of 1 : 1.2 increased the amount of spherical Sb-rich Sb2Se3 particles, which showed enhanced catalytic activities. The hollow spherical Sb-rich Sb2Se3 particles were found to exhibit much better catalytic performances than the rod-shaped Se-rich Sb2Se3, which may be attributed to the higher dispersity of antimony and the small sizes of the hollow spherical Sb-rich Sb2Se3 particles (less than 50 nm). The potential growth mechanisms for the different morphologies of Sb-rich Sb2Se3 have been proposed by analysing the samples at different growth stages.

 Please wait while we load your content...
Please wait while we load your content...