Reduced graphene oxide as a highly efficient adsorbent for 1-naphthol and the mechanism thereof†
Abstract
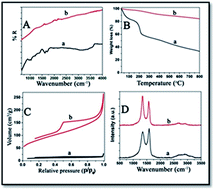
Herein, adsorption and removal of 1-naphthol from water using reduced graphene oxide (rGO) is reported. The adsorption isotherms of different rGOs at different temperatures (303 and 323 K) fit well with Langmuir adsorption isotherms. At 303 K, the maximum adsorption capacities (qmax) of the relatively lower and higher surface area rGOs (l-rGO and h-rGO) are 3.57 and 5.55 mmol g−1 respectively. The qmax for the adsorption of 1-naphthol by h-rGO, at 323 K, is a remarkable 8.6 mmol g−1, which is the highest so far reported in the literature. The qmax value is greater than that of the h-rGO's water dispersible graphene counterpart reported in the literature. Acidic and alkaline pH increases the adsorption. Examination of the adsorption mixture using atomic force microscopy shows that the rGO is dispersed as few layered graphene nanosheets. The remarkably higher qmax is attributed to the higher surface area of rGO in aqueous solution due to its exfoliation by 1-naphthol. In summary, rGO is a promising material for the removal of 1-naphthol from water and our findings help in better understanding the mechanism of the adsorption of 1-naphthol and similar compounds by rGO.

 Please wait while we load your content...
Please wait while we load your content...