Comparative study of optical, structural and electrical properties of zinc phthalocyanine Langmuir–Blodgett thin film on annealing
Abstract
Zinc phthalocyanine (ZnPc) in the mixture of N-methyl pyrroldione (NMP) and chloroform (CHCl3) [1 : 10 (v/v)] spreading solvent was observed to have only a monomeric absorption peak. However, on processing the solution into a thin film using the Langmuir–Blodgett (LB) technique, it shows absorption peaks corresponding to H, J and monomer aggregates, as confirmed by UV-Vis absorption spectroscopy. The Q-band absorption spectra of ZnPc LB thin film was observed to have a sharp change on annealing at 65 °C, indicating a change in the aggregation configuration of molecules over the surface. The annealed ZnPc LB thin film is found to have H and monomer aggregates only, which indicates that ZnPc molecules are arranged in an edge on face-to-face conformation. The ZnPc LB thin film remains in this aggregation until the higher annealing temperature. The ZnPc LB thin film shows an α-phase characteristic when annealed at 65 °C, and it remains intact in this phase up to 200 °C. The α–β phase transformation starts occurring from 200 °C and is completed at 290 °C. The large increase in crystallite size, as obtained from the XRD study, and the change in the shape of the ZnPc nanoparticles from spherical to nanorod structure, as observed from FESEM and AFM images, confirms the transformation of the ZnPc film from metastable α phase to stable β phase. Electrical conductivity is found to be enhanced considerably for β-phase with respect to α-phase in dark and under photoexcitation because of better charge carrier transport.
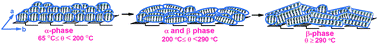

 Please wait while we load your content...
Please wait while we load your content...