Low-temperature sol–gel synthesis of crystalline materials
Abstract
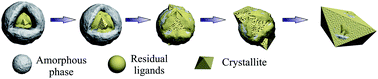
Sol–gel chemistry has opened a new era of modern materials science by enabling the production of ceramic materials at near-room temperature. Thousands of papers have been published since its inception, and new hybrid materials and composites widely used in our everyday life have been obtained. From a chemical point of view, these materials actually have compositions identical to their high-temperature ceramic analogs, but there is a drastic difference in structure and phase composition. In the majority of cases, oxide systems produced using the sol–gel method possess an amorphous structure and huge surface area with narrow micro/mesopore size distribution. At the same time, there are a great variety of oxides and mixed-oxide systems with quite a number of polymorphic modifications and, consequently, certain properties can only be produced by high-temperature treatment. Investigation of the mechanisms and methods of crystallization for such systems in the colloidal state at temperatures less than 100 °C would significantly contribute to the development of new materials obtained by low-temperature sol–gel synthesis. Taking into account the millions of different thermosensitive organic, inorganic, and bio-organic substances that could be used in producing hybrids and composites, the potential of low-temperature sol–gel technology is immense. In fact, it is a ‘second wind’ for developing classical sol–gel technology, with its more than a hundred-year history. The present review describes the fundamental principles of crystallization of oxide sol–gel systems in solution and gives examples of the applications of composites produced by low-temperature sol–gel synthesis.

 Please wait while we load your content...
Please wait while we load your content...