Design, synthesis, and biological evaluation of a new class of MT2-selective agonists†
Abstract
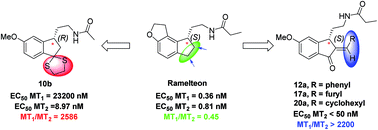
A novel class of chiral 2,3-dihydro-1H-indene derivatives were designed and synthesized as melatonergic ligands. Most of the reported MT2-selective ligands behave as antagonists. By contrast, our exploration of 2,3-dihydro-1H-indene showed that the introduction of a lipophilic group at the 2- or 3-position of this scaffold could afford highly selective MT2 agonists. Among all these synthesized molecules, compounds 10b, 12a, 17a, 20a exhibited powerful MT2 agonistic activity (EC50 < 50 nM) as well as excellent MT2 selectivity (more than 2200-fold).

 Please wait while we load your content...
Please wait while we load your content...