9-Iodophenalenone and 9-trifluoromethanesulfonyloxyphenalenone: convenient entry points to new phenalenones functionalised at the 9-position. Iodine-carbonyl interaction studies by X-ray crystallography†
Abstract
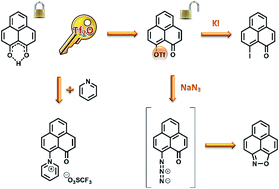
Reaction of 9-hydroxyphenalenone 1 with triflic anhydride allows access to 9-trifluoromethanesulfonyloxyphenalenone 2. The reactivity of 2 is explored in the reactions with pyridine, iodide, and azide. Structural aspects of 4, including carbonyl–iodine interactions, are investigated by X-ray crystallography. Both 2 and 9-iodophenalenone 4 are expected to be valuable precursors in the synthesis of further 9-functionalised phenalenones.

 Please wait while we load your content...
Please wait while we load your content...