Kinetic resolution of citronellal by chiral aluminum catalysts: l-menthol synthesis from citral†
Abstract
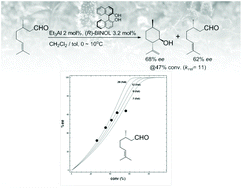
A highly reactive catalytic ring-closing ene reaction is discussed. This reaction is catalyzed via novel optically active aluminum BINOL and TADDOL complexes. The kinetic resolution of the racemic analogs of citronellal was affected by these Al catalysts. The BINOL-Al catalyst afforded 68% ee of a diastereomer of isopulegol and 62% ee of citronellal at 47% conversion. The reaction mechanism proposed assumes that the optically active catalyst possesses a metal center between two parallel aromatic rings. We postulate that the edge of the aromatic rings can recognize the methyl group at the 3-position of citronellal, as the rings are oriented in a pseudoparallel orientation. We utilized the kinetic resolution for the synthesis of L-menthol from citral.

 Please wait while we load your content...
Please wait while we load your content...