Living syndiospecific polymerization of propylene with sterically encumbered titanium complexes activated by MMAO†
Abstract
A series of sterically encumbered (salicylaldiminato)(β-enaminoketonato)titanium complexes [3-tBu-2-OC6H3CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N(C6F5)][RN
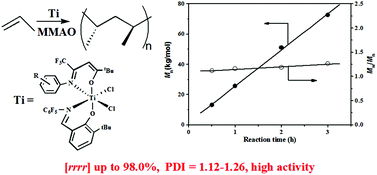
N(C6F5)][RN![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C(CF3)CHC(tBu)O]TiCl2 [1a: R = Ph, 1b: R = C6H4F(p), 1c: R = C6H4Cl(p), 1d: R = C6H4Br(p), 1e: R = C6H4Br(o)] were synthesized and tested to be efficient catalysts for syndiospecific polymerization of propylene in the presence of modified methylaluminoxane at room temperature. The introduction of a bulky bromine atom ortho to the imine nitrogen of the β-enaminoketonato ligand not only successfully improved the pentad syndiotacticity (rrrr) of the resulting polypropylenes from 88.5% to 97.2%, but also provided better protection of the active site from attack of free AlR3 or monomers and thus contributed to the living polymerization nature, while keeping high catalytic activity. More importantly, compared with the famous pentafluorinated FI-Ti/MAO catalyst system, the sterically congested complex 1e with the bromine atom ortho to a N-aryl group displayed almost two times higher catalytic activity (14.5 vs. 28.0 kg mol−1 h−1), producing polypropylenes with even higher pentad syndiotacticity (rrrr = 97.2% vs. 96.0%) and similar narrow molecular weight distributions (Mw/Mn = 1.12–1.26). In addition, the polymerization proceeded with a different monomer insertion mode of 1,2-insertion and a similar chain-end control mechanism. Quantitative 13C NMR spectra revealed that polymers with various stereo structures ranging from highly syndiotactic and regioregular to atactic and regio-irregular polymers at different reaction temperatures were achieved, and the probable formation routes were proposed. The percentage of regio-irregularities of the monomer sequence arising from 2,1-insertion and 3,1-enchainment increased with the rise of reaction temperature.
C(CF3)CHC(tBu)O]TiCl2 [1a: R = Ph, 1b: R = C6H4F(p), 1c: R = C6H4Cl(p), 1d: R = C6H4Br(p), 1e: R = C6H4Br(o)] were synthesized and tested to be efficient catalysts for syndiospecific polymerization of propylene in the presence of modified methylaluminoxane at room temperature. The introduction of a bulky bromine atom ortho to the imine nitrogen of the β-enaminoketonato ligand not only successfully improved the pentad syndiotacticity (rrrr) of the resulting polypropylenes from 88.5% to 97.2%, but also provided better protection of the active site from attack of free AlR3 or monomers and thus contributed to the living polymerization nature, while keeping high catalytic activity. More importantly, compared with the famous pentafluorinated FI-Ti/MAO catalyst system, the sterically congested complex 1e with the bromine atom ortho to a N-aryl group displayed almost two times higher catalytic activity (14.5 vs. 28.0 kg mol−1 h−1), producing polypropylenes with even higher pentad syndiotacticity (rrrr = 97.2% vs. 96.0%) and similar narrow molecular weight distributions (Mw/Mn = 1.12–1.26). In addition, the polymerization proceeded with a different monomer insertion mode of 1,2-insertion and a similar chain-end control mechanism. Quantitative 13C NMR spectra revealed that polymers with various stereo structures ranging from highly syndiotactic and regioregular to atactic and regio-irregular polymers at different reaction temperatures were achieved, and the probable formation routes were proposed. The percentage of regio-irregularities of the monomer sequence arising from 2,1-insertion and 3,1-enchainment increased with the rise of reaction temperature.

 Please wait while we load your content...
Please wait while we load your content...