Robust asymmetric synthesis of unnatural alkenyl amino acids for conformationally constrained α-helix peptides†
Abstract
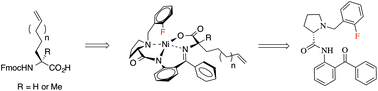
The efficient asymmetric synthesis of unnatural alkenyl amino acids required for peptide ‘stapling’ has been achieved using alkylation of a fluorine-modified NiII Schiff base complex as the key step.
 Please wait while we load your content...
Please wait while we load your content...