Azabicycles construction: the transannular ring contraction with N-protected nucleophiles
Abstract
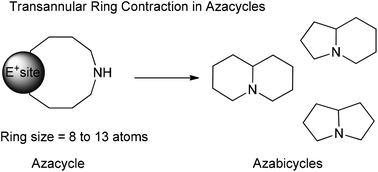
Synthetic strategies are one of the most critical factors for the success of a synthetic campaign, but most importantly they are crucial for the economy and the efficiency of the sequence. Within this perspective, the synthesis of azabicyclic scaffolds, constituents of many bioactive alkaloids, can be tackled using tandem transannular ring contractions-protecting group cleavages. Commonly these reactions feature medium-sized cyclic N-protected amines that carry a potential electrophilic site. Under the correct conditions this site is activated, and the protecting group is fragmented, inducing a transannular reaction. For the first time, we review in detail this strategy's applications, which span from the assembly of small molecules to complex molecular architectures.

 Please wait while we load your content...
Please wait while we load your content...