Stereoselective synthesis of O-tosyl azabicyclic derivatives via aza Prins reaction of endocyclic N-acyliminium ions: application to the total synthesis of (±)-epi-indolizidine 167B and 209D†
Abstract
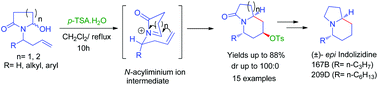
A diastereoselective protocol has been established for the synthesis of 4-O-tosyl piperidine containing hexahydroindolizin-3(2H)-one, hexahydro-1H-quinolizin-4(6H)-one and 1,3,4,10b-tetrahydropyrido[2,1-a]isoindol-6(2H)-one derivatives via the aza-Prins cyclization reaction of cyclic N-acyliminium ions mediated by p-toluene sulphonic acid (p-TSA) under mild conditions. The reaction is highly diastereoselective and gives excellent yields. This method has been applied to an efficient total synthesis of indolizidine alkaloids, (±)-epi-indolizidine 167B and 209D.

 Please wait while we load your content...
Please wait while we load your content...