Copper-catalyzed direct alkylation of 1,3-azoles with N-tosylhydrazones bearing a ferrocenyl group: a novel method for the synthesis of ferrocenyl-based ligands†
Abstract
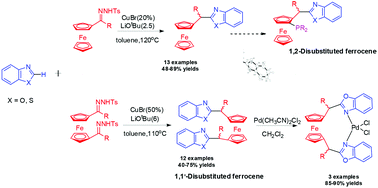
Copper-catalyzed cross-coupling of ferrocenyl ketone-derived N-tosylhydrazones with benzo[d]oxazole leads to the direct C–H bond functionalization by a secondary ferrocenyl alkyl group. This direct C–H bond alkylation of azoles with N-tosylhydrazones bearing a ferrocenyl group uses inexpensive CuBr as the catalyst without any ligand. The reaction is operationally simple and conducted under mild conditions, giving the corresponding ferrocenyl-based ligands in moderate to good yields. Furthermore, they were able to act as bidentate ligands, giving rise to the corresponding palladium chelated complex 6a–6c, which were obtained by reaction of 5a–5c with [PdCl2(MeCN)2].

 Please wait while we load your content...
Please wait while we load your content...