Isovanillin derived N-(un)substituted hydroxylamines possessing an ortho-allylic group: valuable precursors to bioactive N-heterocycles†
Abstract
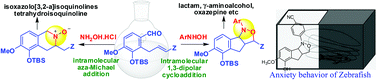
The intramolecular 1,3-dipolar cycloaddition of isovanillin derived N-aryl hydroxylamines possessing ortho-allylic dipolarophiles affords novel benzo analogues of tricyclic isoxazolidines that can be readily transformed into functionalized lactams, γ-aminoalcohols and oxazepines. The corresponding N-unsubstituted hydroxylamines give rise to tetrahydroisoquinolines. Anxiogenic properties of these compounds are tested in zebra fish.

 Please wait while we load your content...
Please wait while we load your content...